Genome editing has progressed rapidly from discovery to clinical development, while preclinical studies continue to refine the approaches. Now, two papers in Nature Medicine showcase the potential of two such strategies — base editing and CRISPR–Cas9 — for prevention of hypertrophic cardiomyopathy (HCM) in mouse models. The studies also highlight different advantages and challenges for each genome-editing strategy.
HCM is caused by mutations in cardiac sarcomeric genes that lead to thickening of the heart muscle and can cause heart failure and sudden cardiac death. A dominant-negative pathogenic variant in the sarcomeric protein β-myosin, c.1208G>A, is a well-studied cause of severe HCM.
In one of the studies, Chai et al. set out to correct the c.1208G>A mutation using a base editor — a fusion protein consisting of a modified Cas9 nickase and a deaminase enzyme that converts one DNA base into another, at a site determined by a guide RNA (gRNA) sequence.
The authors used human induced pluripotent stem cells (iPSCs) to screen various adenine base editors (ABEs) with different editing efficiency and specificity. They opted for an ABE with a narrow editing window. Although this had a relatively low efficiency (34%), the risk of bystander edits — whereby the base editor modifies other adenine residues close to the target adenine — was also low.
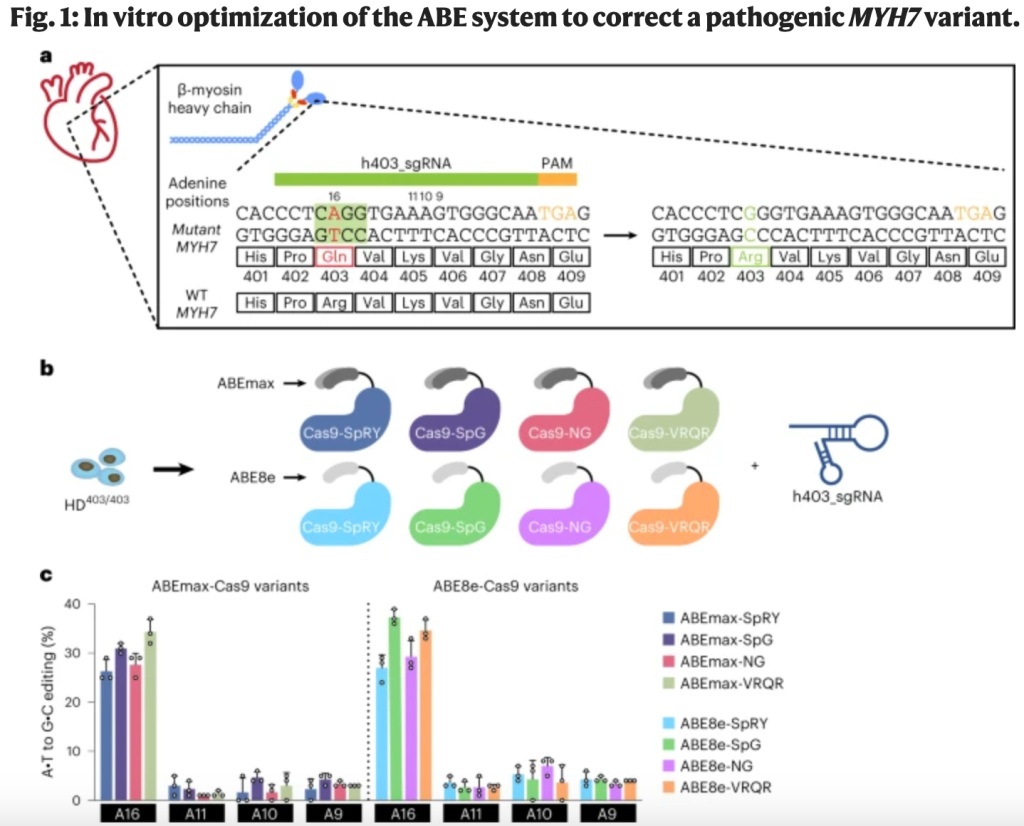
In cardiomyocytes generated from patient-derived iPSCs heterozygous for the c.1208G>A mutation, treatment with the base editor reduced contractile force generation and ATP consumption back to normal levels. The researchers detected minimal bystander editing with little to no off-target editing at distant DNA sites.
Next, Chai et al. generated a mouse model that carried human sequences encoding the β-myosin pathogenic variant. Owing to the large size of the base editor enzyme, they delivered it over two adeno-associated virus 9 (AAV9) vectors, and used a troponin T promoter to target expression to cardiomyocytes.
Mice received intrathoracic injection of the AAV9-vectored base editor immediately after birth, prior to development of HCM. Compared with untreated mice, at 8–16 weeks ABE-treated mice showed reduced features of HCM, such as ventricular wall thickening, and had similar echocardiographic readouts to wild-type mice.
The investigators detected a 32% editing efficiency of the target pathogenic adenine in cardiomyocytes, with no bystander editing. Low-level editing in off-target tissue such as the liver was detected.
In the other study, Reichart et al. tested both an ABE and a CRISPR–Cas9 nuclease that would respectively correct or silence the β-myosin mutation.
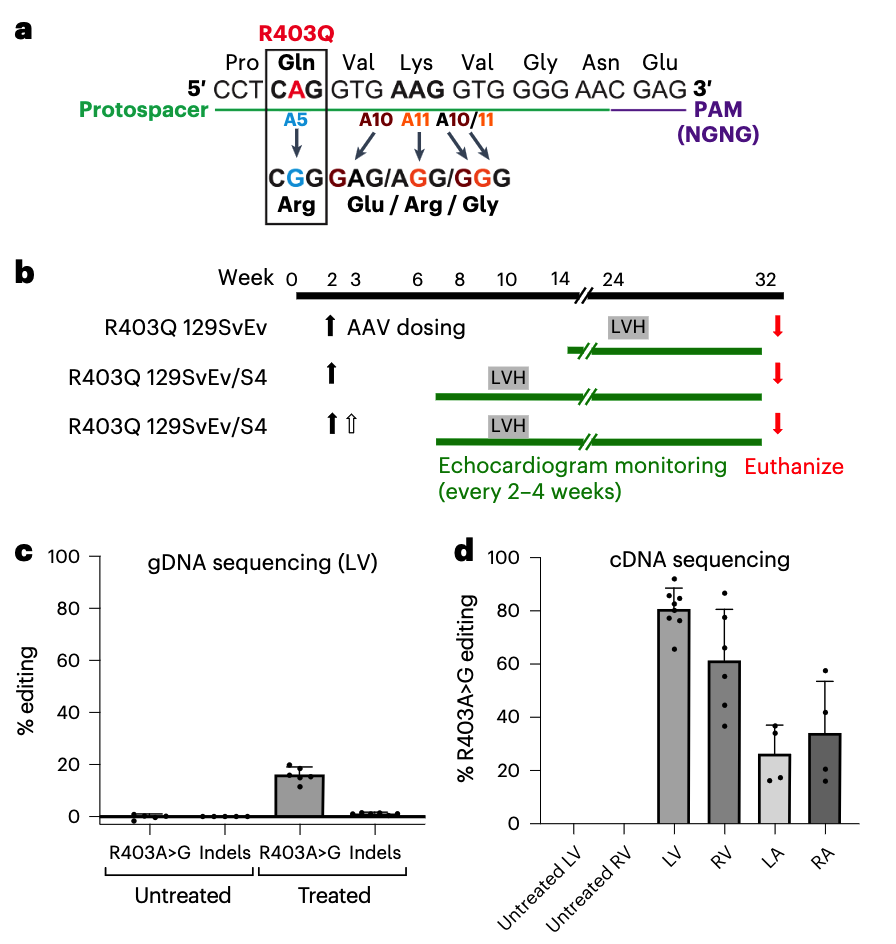
They selected an ABE with high editing efficiency, which corrected the mutation in >70% of left ventricular cardiomyocytes. Treatment of mouse models at 10–13 days of age prevented onset of HCM cardiac morphology and dysfunction for 32 weeks. However, bystander edits occurred at a rate of 3–5%, and a low but significant rate of off-target editing was detected.
Reichart et al. also designed a CRISPR–Cas9 nuclease system to selectively inactivate the c.1208G>A mutation in cardiomyocytes. Here, the gRNA directed Cas9 nuclease to make double-stranded breaks (DSBs) and generate indels in the target gene. The smaller size of Cas9 compared with ABEs enables the therapeutic to be packaged in a single AAV. And although this approach cannot correct a mutation, it is more amenable to application across different mutations.
Intrathoracic injection of the CRISPR–Cas9 prevented HCM onset in mouse models. However, high-dose treatment was associated with impaired cardiac function resulting from editing of the wild-type allele, suggesting a narrow therapeutic window.
Further studies and emerging clinical data will assist researchers in balancing editing efficiency with safety and other parameters to select appropriate genome-editing tools.
1- Chai, A. C. et al. Base editing correction of hypertrophic cardiomyopathy in human cardiomyocytes and humanized mice. Nat. Med. 29, 401–411 (2023)
2- Reichart, D. et al. Efficient in vivo genome editing prevents hypertrophic cardiomyopathy in mice. Nat. Med. 29, 412–421 (2023)