AAV-mediated gene-directed therapies hold great promise, but success is contingent on effective and safe transduction of the target tissue. This constitutes a particular challenge for the largest organ, skeletal muscle. In this issue of Cell, a study by Tabebordar et al. (2021) describes an ingenious method of directed capsid evolution generating a novel class of muscle-specific capsids that allow for lower and thus safer therapeutic doses. If successfully translated to human, this discovery along with the study by Weinmann et al. (2020) has the exciting potential to make muscle directed gene therapy safer, more effective, and more attainable.
Inherited disorders of skeletal muscle contribute significantly to genetic morbidity and mortality at all stages of life. Gene- and transcript-directed therapies are moving firmly toward the clinic, offering hope for a new therapeutic era of genetic medicines for these hitherto intractable disorders, with clinical trials of AAV-mediated gene therapy ongoing in individuals with Duchenne muscular dystrophy (DMD) (Duan, 2018), forms of limb-girdle muscular dystrophy, X-linked myotubular myopathy (XLMTM), and Pompe disease.
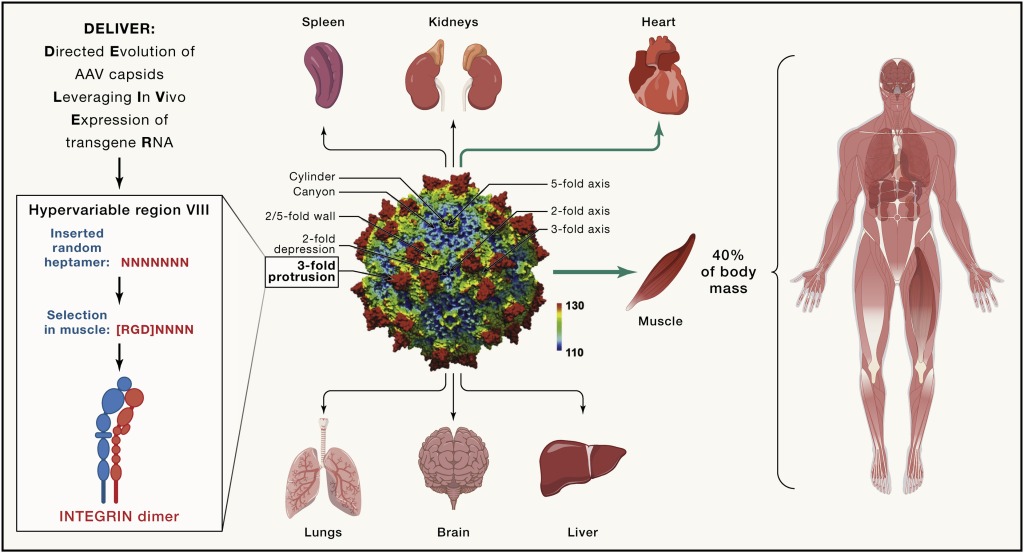
The systemic muscle-directed gene therapies currently use the natural AAV serotypes AAV8, AAV9, and AAVrh74. While they also target both skeletal and cardiac muscle, they are not selective and prominently target the liver. Skeletal muscle is unique in that it is the organ with the largest mass (about 40% of body mass) and an extremely wide anatomical distribution. To effectively target all relevant muscles for movement, breathing, and cardiac function, these serotypes currently used require very high systemic doses. With that, serious toxicities are now emerging in several of the muscle-directed clinical trials, resulting from the considerable and undesired targeting of the liver as well as from immunological issues, in particular complement system activation (Paulk, 2020). Such high doses also impose considerable strains on product manufacturing, resulting in scarcity and high costs.
In contrast, a capsid with specifically increased tropism to muscle while de-emphasizing the liver would allow for lower doses and decreased toxicities. Most of the tropism of AAV is dependent on cell receptor-binding epitopes of the AAV capsid, the functional landscape of which is increasingly understood (Wang et al., 2019). Along with rational in silico design of AAV capsids and new natural capsid discovery, innovative methods have been developed to empirically identify novel capsids with desired tropism based on evolutionary considerations, capsid shuffling, and directed evolution (Herrmann et al., 2019; Maheshri et al., 2006; Zinn et al., 2015) and others (Wang et al. [2019] for review). In the latter approach, new capsid libraries are generated by random peptide permutation within the hypervariable regions in the 3-fold protrusion of the capsid to modify tropism while preserving basic properties of AAV. This has led to promising developments such as the CREATE protocol, which was successfully applied for CNS targets (Deverman et al., 2016).
Two studies, by Weinmann et al. (Weinmann et al., 2020) and by Tabebordar et al. (Tabebordar et al., 2021), have been remarkably successful in achieving this for skeletal muscle. Weinmann et al. arrived at a highly myotropic capsid via a secondary in vivo screen of a preselected number of promising capsids using barcoded libraries to be screened at both the DNA and RNA levels. The ingenious approach taken by Tabebordar et al. involves screening the entire spectrum of randomly varied heptapeptide inserted in the hypervariable region VIII of AAV9, followed by an in vivo selection method, which, importantly, allows for screening of the entire randomly generated library in any strain or species. Using a muscle-specific promoter to drive the actual sequence of each AAV’s individual capsid as the “barcode,” specific targeting to muscle is selected for, requiring transduction, unpacking, and transcription in muscle. RNA sequencing of transcribed capsid sequences allows for recovery of muscle-enriched capsids out of the highly complex library. The authors refer to this method as DELIVER (directed evolution of AAV capsids leveraging in vivo expression of transgene RNA) (Figure 1).
Initially selected for in mice, this approach identified a capsid family that targeted muscle (and the heart) strikingly more efficiently as compared with the natural serotypes, with decreased targeting of the liver. This allowed for substantially lower systemic doses to achieve the desired therapeutic effect relative to the new capsids (referred to as MyoAAV): two traditional capsids in the myotubularin inactivation model (for XLMTM) delivering gene replacement and the mdx mouse model (for DMD) delivering gene editing. Remarkably, as earlier shown by Weinmann et al., the selected capsid family encoded an RGD integrin binding motif as their commonality. The muscle-targeting effect was at least partly dependent on integrin binding, offering a mechanistic window into the new tropism. It is encouraging for the muscle-targeting field and for rational capsid design that an RDG motif also emerged in the Weinmann study.
A caveat is how well these new tropisms will translate across species and strains (Hordeaux et al., 2019). Because of the easy species portability of the DELIVER protocol, it was possible to screen directly in a non-human primate (NHP), again arriving independently at a similar capsid with an RGD motif. This new NHP-derived iteration of MyoAAV appeared to be even better at muscle targeting, bringing potential translation to human within reach.
Preclinical rigor in the development of new capsids must be high, as the path to clinical translation remains arduous and expensive. Will it produce and package efficiently at the required clinical scale with preserved stability and potency? Would natural AAV9 seropositivity preclude dosing in human (AAV9 antibodies bind MyoAAV)? Evaluation of toxicity specifically in human is paramount, as the complement toxicity for instance was not predicted preclinically. Unpredicted toxicities from other organs and systems may also emerge. Still, the much lower systemic doses required (if translatable to human) will go a long way in mitigating any such systemic toxicities.
The feasibility and independent reproducibility of identifying bespoke capsids bodes well for gene-directed medicine, as its precision can be extended to the delivery tools. Given that all gene therapy is dependent on effective and safe delivery, the importance of this development is obvious.
REFERENCES
Deverman, B.E., Pravdo, P.L., Simpson, B.P., Kumar, S.R., Chan, K.Y., Banerjee, A., Wu, W.L.,
Yang, B., Huber, N., Pasca, S.P., and Gradinaru, V. (2016). Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209.
Duan, D. (2018). Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy.Mol. Ther. 26, 2337–2356.
Herrmann, A.K., Bender, C., Kienle, E., Grosse,S., El Andari, J., Botta, J., Schu¨rmann, N.,
Wiedtke, E., Niopek, D., and Grimm, D. (2019).A Robust and All-Inclusive Pipeline for Shuffling
of Adeno-Associated Viruses. ACS Synth. Biol.8, 194–206.
Hordeaux, J., Yuan, Y., Clark, P.M., Wang, Q., Martino, R.A., Sims, J.J., Bell, P., Raymond, A., Stanford, W.L., and Wilson, J.M. (2019). The GPI-LinkedProtein LY6A Drives AAV-PHP.B Transport acrossthe Blood-Brain Barrier. Mol. Ther. 27, 912–921
Maheshri et al., 2006
N. Maheshri, J.T. Koerber, B.K. Kaspar, D.V. Schaffer Directed evolution of adeno-associated virus yields enhanced gene delivery vectors Nat. Biotechnol., 24 (2006), pp. 198-204
N. Paulk Gene Therapy: It’s Time to Talk about High-Dose AAV. Genetic Engineering & Biotechnology News (2020) https://www.genengnews.com/commentary/gene-therapy-its-time-to-talk-about-high-dose-aav/
S. Pipe, F.W.G. Leebeek, V. Ferreira, E.K. Sawyer, J. Pasi Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene TransferMol. Ther. Methods Clin. Dev., 15 (2019), pp. 170-178
M. Tabebordar, K.A. Lagerborg, A. Stanton, E.M. King, S. Ye, L. Tellez, A. Krunnfusz, S. Tavakoli, J.J. Widrick, K.A. Messemer, et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across speciesCell, 184 (2021), pp. 4919-4938
D. Wang, P.W.L. Tai, G. Gao Adeno-associated virus vector as a platform for gene therapy deliveryNat. Rev. Drug Discov., 18 (2019), pp. 358-378
J. Weinmann, S. Weis, J. Sippel, W. Tulalamba, A. Remes, J. El Andari, A.K. Herrmann, Q.H. Pham, C. Borowski, S. Hille, et al.Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variantsNat. Commun., 11 (2020), p. 5432
E. Zinn, S. Pacouret, V. Khaychuk, H.T. Turunen, L.S. Carvalho, E. Andres-Mateos, S. Shah, R. Shelke, A.C. Maurer, E. Plovie, et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy VectorCell Rep., 12 (2015), pp. 1056-1068