CAS13 BASICS
DNA targeting CRISPR enzymes, such as Cas9 and Cas12a (formerly Cpf1), have enabled many new possibilities for manipulating and studying DNA. Recent computational efforts to identify new CRISPR systems uncovered a novel type of RNA targeting enzyme, Cas13. The diverse Cas13 family contains at least four known subtypes, including Cas13a (formerly C2c2), Cas13b, Cas13c, and Cas13d (Fig. 1a). We originally showed that Cas13a could programmatically bind and cleave RNA, protecting bacteria from RNA phages and serving as a powerful platform for RNA manipulation (Fig. 1b) (Abudayyeh*, Gootenberg*, Konermann* et al Science 2016), but further computational and biochemical exploration of Cas13 has led to an understanding of additional subtypes.

Figure 1. The Cas13 family. A. Four subtypes of CRISPR-Cas13 systems have been reported to date, which are classified based on the identity of the Cas13 protein and additional locus features. All known Cas13 family members contain two HEPN domains (shown in orange), which confer RNase activity. B. Cas13 can be reprogrammed to cleave a targeted ssRNA molecule through a short guide RNA with complementarity to the target sequence.
Cas13s function similarly to Cas9, using a ~64-nt guide RNA to encode target specificity. The Cas13 protein complexes with the guide RNA via recognition of a short hairpin in the crRNA, and target specificity is encoded by a 28 – 30-nt spacer that is complementary to the target region. In addition to programmable RNase activity, all Cas13s exhibit collateral activity after recognition and cleavage of a target transcript, leading to non-specific degradation of any nearby transcripts regardless of complementarity to the spacer.
It was hypothesized that this activity may be part of a programmed cell death pathway in bacteria, allowing cells to commit cell suicide or become dormant unless they recover from infection. Fortunately, the collateral activity is undetectable in mammalian cells and plants allowing for many RNA targeting applications to be developed using Cas13. Many applications have also been built using Cas13s in mammalian cells, including transcript knockdown, live-cell transcript imaging (Abudayyeh*, Gootenberg* et al., Nature 2017), and RNA base editing (Cox*, Gootenberg*, Abudayyeh* et al., Science 2017).
While Cas13a showed some activity for RNA knockdown, certain orthologs of Cas13b proved more stable and robust in mammalian cells for RNA knockdown and editing. More recently, additional orthologs of Cas13 have been discovered, including Cas13d, which has been leveraged for efficient and robust knockdown across many endogenous transcripts (Konermann et al., Cell 2018). Konermann et al. additionally showed that Cas13d can be used to modulate splicing of endogenous transcripts and that the coding sequence for Cas13d is small enough to fit within the packaging limits of AAV for in vivo delivery.
Beyond these in vivo activities, we and others realized that Cas13s non-specific RNase activity, could be leveraged to cleave fluorescent reporters upon target recognition, allowing for the design of sensitive and specific diagnostics using Cas13. We dubbed our specific detection system SHERLOCK (Gootenberg*, Abudayyeh* et al., Science 2017).
Below, we discuss tips and tricks for a variety of Cas13 applications, including knockdown, RNA editing, and SHERLOCK, and include a discussion about how to choose between the variety of Cas13 orthologs.
KNOCKDOWN WITH CAS13
One of the most straightforward applications of Cas13 in vivo is targeted RNA knockdown using mammalian codon optimized Cas13 and guide expression vectors. Knockdown of RNA (Fig. 2a) relies on cleavage of the targeted transcripts by the endogenous RNase activity of the dual HEPN domains of the protein, the efficiency of which varies between different orthologs and subtypes of Cas13 (see Table 1). As a result, guide design and restrictions on targeting depend on the system used.
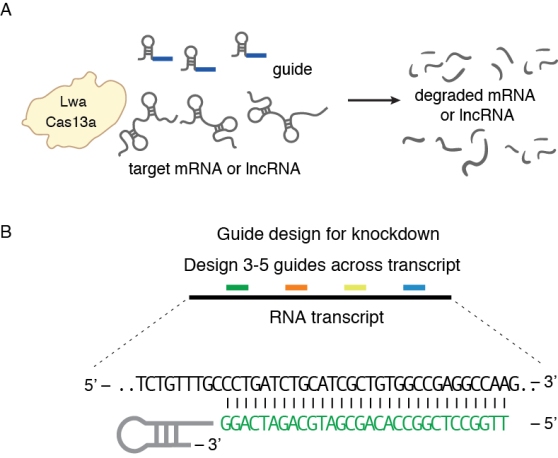
Figure 2. Knockdown of target RNA molecules with Cas13
● Cas13 ortholog-specific guide restrictions
In the initial characterization of Cas13a (Abudayyeh*, Gootenberg*, Konermann* et al., Science 2016), the Cas13a from Leptotrichia shahii (LshCas13a) demonstrated a sequence constraint, termed the Protospacer Flanking Sequence (PFS), both in vitro as well as in bacterial cells. Immunization of E. coli against MS2 phage by heterologous expression of the LshCas13a system depended on the presence of a 3’ H (not G) base immediately flanking the protospacer sequence. Similar PFS restrictions have been observed in bacteria for Cas13b (Smargon*, Cox*, Pyzocha* et al., Molecular cell 2017;Cox*, Gootenberg*, Abudayyeh* et al., Science 2017). However, in mammalian cells, no PFS restriction has been documented for the Cas13 orthologs tested, including Cas13a, Cas13b, and Cas13d. Additional sequence-based rules for targeting with Cas13 in vivo will likely be deduced as the number of guides tested increases over time.
● Target restrictions due to RNA secondary structure
An additional factor that must be considered when targeting single-stranded RNA species in vivo is the accessibility of the target due to higher-order structures. In vitro, Cas13 cleaves near unstructured regions of the target RNA (Abudayyeh*, Gootenberg*, Konermann* et al., Science 2016), and in vivo, predicted secondary structure (i.e., double-stranded RNA) is negatively correlated with knockdown in bacteria or mammalian cells, as Cas13 requires a single-stranded substrate and likely lacks the helicase activity necessary for opening up double-stranded RNA regions for guide binding.
In bacteria, screening guides against E. coli essential genes with Cas13b revealed that spacers with secondary structure near the protospacer had reduced depletion in the screen (Smargon*, Cox*, Pyzocha* et al Molecular cell 2017). In mammalian cells, spacers tiled along the length of target transcripts resulted in greater knockdown of the transcript when targeted in regions of low secondary structure (Abudayyeh*, Gootenberg* et al., Nature 2017). Although these associations are not definitive, targeting regions with substantial base pairing may reduce the efficiency of knockdown. In both studies, the RNAplfold algorithm was used (Bernhart et al., 2006), although for ease of use online tools such as RNAfold can provide computational folding predictions. Additionally, the siRNA design tool RNAxs can be used for finding regions of a transcript that have good accessibility to narrow the target region space for designing crRNAs. Whether targeting accessible regions or not, we usually test 3-5 guides to find a highly efficient one (Fig. 2b). Future dedicated Cas13 design tools will streamline Cas13 knockdown experiments.
Table 1. Comparison of top Cas13 orthologs for RNA knockdown in mammalian cells.
Ortholog Organism Efficiency Reference
LwaCas13a Leptotrichia wadeii ~50% knockdown on luciferase and endogenous transcripts Abudayyeh et al. Cox et al.
RfxCas13d Ruminococcus flavefaciens 80-95% on mCherry reporter and endogenous transcripts Konermann et al.
● Differences in Cas13 subtype activity in different model systems
Beyond the above design guidelines, the many different Cas13 subtypes have varied activities in different model systems. Because of the variety of Cas13 subtypes and orthologs, selection of the right construct for knockdown can be difficult. While LwaCas13a, PspCas13, and RfxCas13d have all been demonstrated to achieve robust knockdown across numerous genes in mammalian cells, a comparison between Cas13a, Cas13b, and Cas13d indicated that RfxCas13d has the most robust and substantial knockdown in HEK293T cells (Konermann et al., Cell 2018). For plant applications, only LwaCas13a has been tested, where it achieved substantial knockdown in rice protoplasts.
● Differences in Cas13 subtype activity in different model systems
Beyond the above design guidelines, the many different Cas13 subtypes have varied activities in different model systems. Because of the variety of Cas13 subtypes and orthologs, selection of the right construct for knockdown can be difficult. While LwaCas13a, PspCas13, and RfxCas13d have all been demonstrated to achieve robust knockdown across numerous genes in mammalian cells, a comparison between Cas13a, Cas13b, and Cas13d indicated that RfxCas13d has the most robust and substantial knockdown in HEK293T cells (Konermann et al., Cell 2018). For plant applications, only LwaCas13a has been tested, where it achieved substantial knockdown in rice protoplasts.
RNA EDITING WITH CAS13 (REPAIR)
Another valuable application of RNA targeting is to guide RNA editing enzymes to transcripts for precise base editing. Using Cas13, we developed a programmable RNA editing system that allows for temporal modulation of genetic variants in transcripts. The system, called RNA Editing for Programmable A to I Replacement (REPAIR), works by fusing the adenosine deaminases acting on RNA (ADAR2) deaminase domain to Cas13b (Fig. 3a). Because the optimal substrate for RNA editing activity is a dsRNA template, we are able to direct ADAR2 activity via guide RNA duplex formation at the target site. Previous studies of ADAR function have shown that the deaminase activity is dependent on the duplex length and position of the targeted adenine within the duplex. While there are not strict guide design rules for REPAIR yet, we have found that generally 50-nt guides work better than longer guides (e.g., 70 or 84 nt in length). In some cases 30-nt guides work best, but as a general rule of thumb, 50-nt guides will provide the most robust editing efficiency.
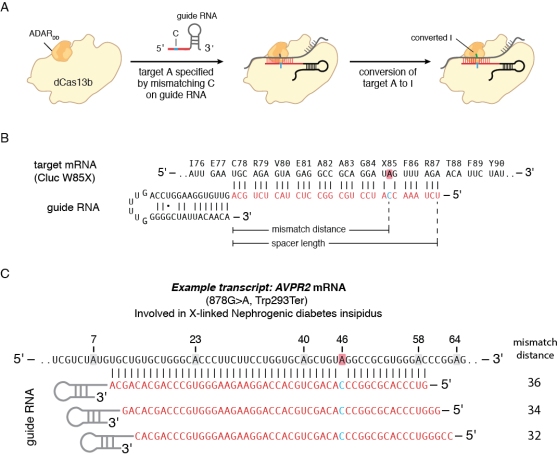
Figure 3. RNA Editing with Cas13 (REPAIR).
In our initial work, it seemed as if putting the target base in the 34th nucleotide position would reliably provide the highest editing efficiency (Fig. 3b). In practice, it is best to test several different designs placing the base pair in positions 32-36 in order to find the optimal design for editing (Fig. 3c). Eventually, guide design tools based on large datasets of REPAIR targeting will allow for guide efficacy prediction and easier design.
● Choosing between REPAIRv1 and REPAIRv2
Our initial iteration of REPAIR, version 1 (REPAIRv1), had many off-targets due to its high activity and overexpression. We found that by generating mutants in the ADAR2 catalytic site, we could lower the off-targets by two orders of magnitude and still retain on-target editing (10%-40% editing) with REPAIRv2. As we find that REPAIRv2 does have lower efficiency editing, we find it more appropriate for settings where off-targets could be problematic. If off-targets are not of serious concern, we recommend using REPAIRv1 in order to achieve the highest possible editing rate, such as in most cell types, in vitro or in vivo, that do not show toxicity due to off target effects. In our own experiments, we have tested a handful of cell types that did not experience toxicity due to REPAIR off-targets.
More sensitive cell types such as embryonic stem cells, for example, may need a more delicate approach with REPAIRv2. An alternative approach for lowering the off-target rate is to decrease the expression of the Cas13-ADAR2 fusion while keeping guide expression high. This can be achieved by transfecting less plasmid, using ribonucleoprotein (RNP) complexes, or integrating the fusion into cells. Any of these approaches will lower the protein expression, substantially reducing off-target editing while maintaining high on-target editing.
NUCLEIC ACID DETECTION WITH CAS13 (SHERLOCK)
Beyond applications in cells, certain properties of Cas13 make it well suited for nucleic acid detection. When the enzyme recognizes its target in vitro, it becomes activated and promiscuously cleaves RNA species in solution. This promiscuous and rapid cleavage activity can be used to amplify the signal from as little as 100,000 molecules in solution, equivalent to a femtomolar concentration in a microliter sample. Cas13-based detection is specific, and can be tuned for single-nucleotide distinction at any position on the target.
To engineer an even more sensitive readout, Cas13 detection can be paired with an isothermal pre-amplification step, most commonly recombinase polymerase amplification (RPA), for a completely isothermal process, termed SHERLOCK (Specific High-Sensitivity Enzymatic Reporter unLOCKing) (Fig. 4a). The reaction can be performed either as a two-step reaction, with an RPA reaction followed by a step combining T7 RNA polymerase transcription and Cas13-based detection, or all enzymes can be run simultaneously in a one-pot reaction.
The most difficult part of preparing to run a Cas13-based detection reaction is purifying the Cas13 protein. Unlike in vivo CRISPR tools, the Cas13 protein must be recombinantly expressed and purified. The LwaCas13a protein vector for expression can be found on Addgene, and, if you are equipped with the necessary equipment for protein purification, you can follow our protocol here. Although the LwaCas13a protein is not currently available commercially, feel free to reach out to us for possible sources if you need help acquiring the protein.
When designing a Cas13-based detection experiment, it is common to design the crRNA for the ortholog of choice (typically LwaCas13a) as DNA, and then generate the RNA using an in vitro transcription reaction. Alternatively, guides can be ordered as synthetic RNA from providers such as IDT or Synthego, and we have seen increased performance with synthesized RNA.
When running an RPA pre-amplification, in the case of a complete SHERLOCK reaction, it’s also important to consider primer design to find the primer set with maximal amplification: we typically follow the TwistDx guidelines, and use NCBI Primer blast for primer design (Fig. 4b). Additionally, you must include a T7 RNA polymerase promoter on the forward primer. For maximal chance of success, we design two RPA primer sets and two crRNAs. In most cases, all combinations of the primer sets and crRNAs will work well, but one will have maximal signal and sensitivity.
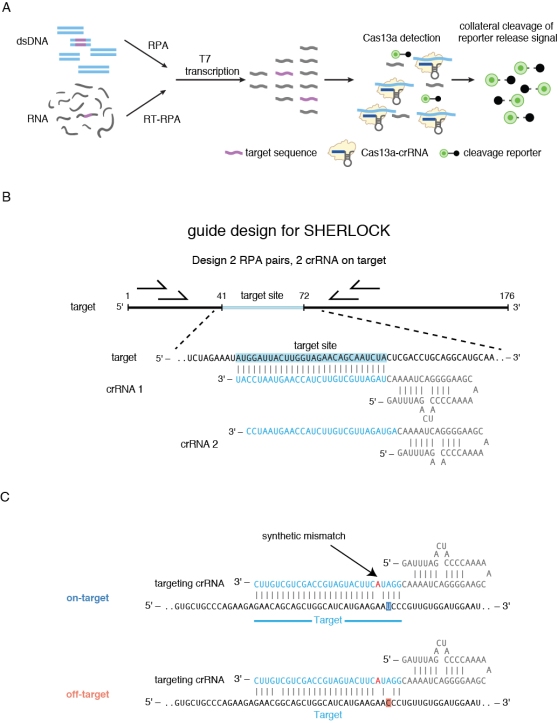
Figure 4. Nucleic acid detection with SHERLOCK.
Additional design rules may apply when implementing other capabilities of SHERLOCK, such as single-nucleotide specificity. We find that we can increase the single-base distinction capabilities of Cas13 by adding an additional mismatch in the guide sequence (i.e., a “synthetic mismatch”) (Fig. 4c). By testing multiple locations, we determined that the optimal placement for the mismatch to be detected is in the third base of the spacer (near the direct repeat), and that the optimal placement for the “synthetic mismatch” was either in position 4 or 5 of the crRNA guide. When testing a mismatch sensitive guide design, we typically test both of these positions to determine the optimal crRNA for maximal distinction.
Recently, we developed additional advancements of the original SHERLOCK technology, including multiplexing using different Cas13 or Cas12 orthologs and lateral flow readout, providing an instrument-free option for point-of-care readouts. Any test can be adapted for lateral flow by purchasing lateral flow strips from providers such as Milenia Biotec, and switching out the fluorescent RNA reporter for a biotin-RNA-FITC reporter molecule. Plasmids for additional Cas13 orthologs for multiplexing can be obtained from Addgene, but, as with Cas13a, the recombinant protein must be purified.